|
Asakura-Oosawa Potential
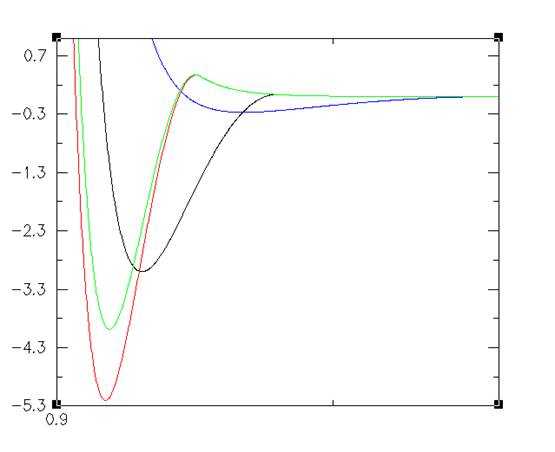
This effect can be compiled by assuming the particles are under the influence of a potential of the form The important parts of this formula are the depth of the potential well and the range of the interaction. The depth of the potential well is determined by the volume fraction of the polymer. The range is determined by the size of the radius of the polymer added to the radius of the colloid.
Figure 1: Energy is on the y axis in kT. Interaction range is on the x axis scaled to the radius of the colloid. Note: Energy is given in terms of kT, where k is the Boltzmann constant and T is an arbitrary temperature. Temperature is arbitrary because the AO interaction is entirely determined by the amount of polymers in the system. More polymers means a stronger interaction, less means a weaker interaction. Temperature does not play a role in the interaction.
|
|
|
|
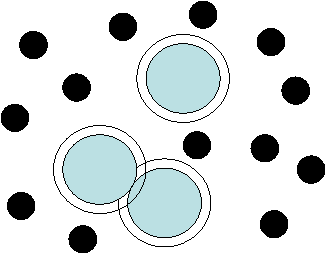 The
AO potential is entirely due to entropy.
The
AO potential is entirely due to entropy.