Dissociation Dynamics of HCO2+
Samuel Diener, Case Western Reserve University, Physics Major
Mentored by Dr. Itzik Ben-Itzhak

I studied the dissociation, breakup without ionization, of HCO₂⁺ when interacting with a high power femto-second laser pulse. Our goals were to see which fragments come out of HCO₂⁺, based on the wavelength used, and the ratio of counts between the fragments.
In order to conduct the experiment, we first needed to extract HCO₂⁺ ions from formic acid, HCOOH, using ECR. If you would like to know more about this, refer to [1] in the references section. From this, we get ions that we can separate using a magnetic field, which deflects the ions based on their charge to mass ratio to our experiment (which can be seen in Fig. 1). Then, our laser, pulsed at 10kHz, hits the ions, causing them to fragment. We are able to separate out the fragments in time by using a spectrometer, electrostatic field towards the detectors. And, we are able to separate out the fragments by position using a deflector, electrostatic field perpendicular to the direction of the detector. In order to detect light fragments, easily accelerated by the deflector, we have a detector we call "Quad" (placed higher), and to catch heavier and neutral fragments we have a detector called "Hex" (placed lower) [2]. These detectors measure the time of arrival of fragments for each laser pulse (event), and the position on the detectors that these fragments hit.
![Fig. 1 - Apparatus, more information can be found at [2] in the references Fig. 1 - Apparatus, more information can be found at [2] in the references](/images/reu/2025/diener/fig1.jpg)
From the events, we are able to develop a coincidence time of flight (CToF) graph, Fig. 2, which matches events from the same laser pulse together. The x-axis of the graph is the time of flight of the first fragment that hits a detector, and the y-axis is the time of flight of the second fragment that hits a detector. This informs us of potential 2-body fragmentations, breakups that result in 2 fragments.
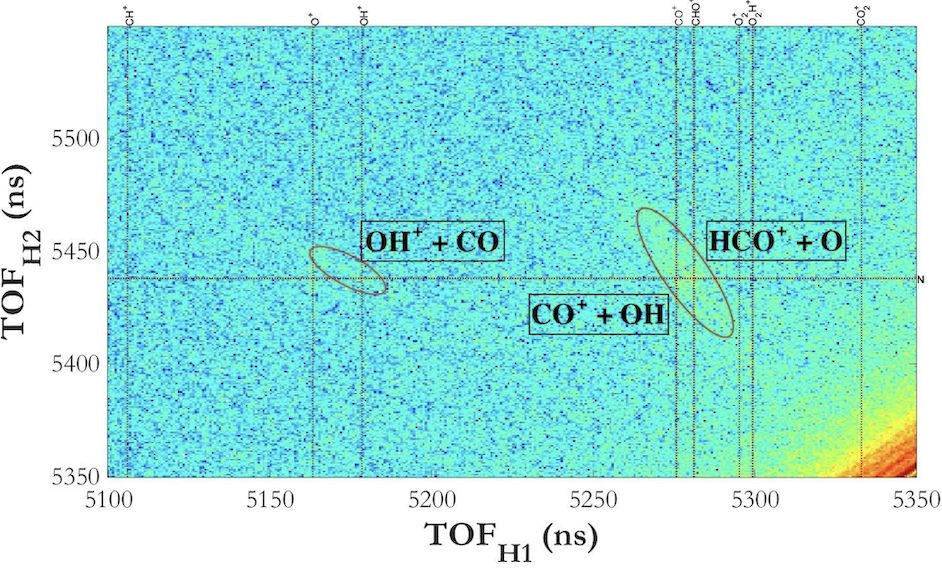
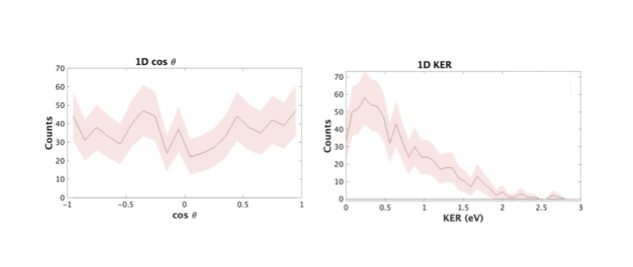
Using kinematic equations, we are able to find the 3-D momenta of potential fragments. And, using momentum conservation, since fragmentation by the laser conserves momentum, we are able to find which events are real and happen consistently. Once we identify channels, or breakup coincidences, we are able to find a few observables, such as kinetic energy release (KER) and angular distributions (referring to the angle between the resulting momentum and polarization of the laser) that result. This can be seen in Fig. 3. These observables weren't a big piece of this experiment since there wasn't anything unusual to report.
We can also measure dissociations for higher body breakup channels, though those were not as prominent in our case. For the next step of the experiment, I used a different wavelength for the laser pulses, allowing me to compare breakup channels for different photon energies. <p> From the experiments, we observed breakup channels: HCO⁺ + O, H⁺ + CO₂, and O⁺ + HCO for 395 nm and 790 nm pulsed experiments. And, we observed the CO⁺ + OH channel for 395 nm experiments. Branching ratios can be made once 3-body breakups are fully analyzed.
Fig. 1.
References
[1] D.K. Olsen, United States, Department of Energy Office of Scientific and Technical Information, DE90 006849, 1989. Information. Introduction to ECR Sources in Electrostatic Machines.
[2] C. Bagdia, T. Severt, N. Iwamoto, A. Filinovich, T. N. Rescigno, A. E. Orel, K. D. Carnes, and I. Ben-Itzhak, Direct Measurement of Charge Transfer Probability during Photodissociation of Few-keV OD+ Beam, The Journal of Physical Chemistry Letters, Vol. 15, 2024, 6859-6865.
Acknowledgments
This material is based upon work supported by the National Science Foundation under Award No. 2244539. I would like to thank the REU Coordinator, Kim Coy, the REU Program Director, Loren Greenman, and the REU Assistant Program Director, Bret Flanders for making this whole program possible and supporting my science communication skills. I would also like to thank Naoki Iwamoto, Nirmallya Das, Vanessa Sanders, and Itzik Ben-Itzhak for all their guidance on the project. And, the James R. Macdonald Laboratory, funded by the Department of Energy, for making it possible, and allowing me, to conduct experiments. And finally, K-State for supporting the program and providing this research opportunity.