




|
|
In order to fully understand how and why aerosol gels are
formed by combustion of an oxidizer with a gas, liquid, or solid a few terms
and explanations are needed. For further information
see…
 |
"Hybrid Superaggregate Morphology as a Result of
Aggregation in a Cluster Dense Aerosol," R. Dhaubhadel,
F. Pierce, A. Chakrabarti, and C.M. Sorensen,
Phys. Rev. E 73, 011404 (2006).
|
 |
"Aerosol Gelation: Synthesis of a Novel, Lightweight,
HighSpecific Surface Area Material" Rajan Dhaubhadel, Corey S. Gerving,
Amitabha Chakrabarti, and Christopher M. Sorensen.
Department of Physics, Kansas State University, Manhattan, Kansas, USA. 01
August 2007 |
..........................................................................................................................................................................................................................................................
 | Aerosol Gel - Quite literally, it is a gel that is formed
in the aerosol phase, through the gelling of its constituents. This is
the key difference between Aero-gel and aerosol gel, aero-gel requires
an all important drying step in order for it to turn into an aero-gel,
aerosol gel does not. Furthermore, the aerosol gel gels in a quick
process from the aggregation of nanometer sized particles that were
formed by the combustion of oxygen and some fuel (gas, liquid, solid
(?)). Ex: 2C2H2 + 202 -> 4C + 2H20.
In this example the carbon would have formed an aerosol gel, i.e. a
carbon aerosol gel. |
 | Gel - A gel is something we interact in our daily lives, be
it hair gel, shampoo, or Jell-O. These gels are formed from a
colloidal solution, where a solid structure has formed a network
throughout its liquid medium. However, aerosol gels are unlike these
gels. Aerosol gels have no liquid medium, they are just a solid
structure of fractal aggregates. |
 | Aggregation - This process is simply the attraction of
particles to one another due to chemical bonding or the Van
der Waals Forces. After the combustion of
a oxidizer and a fuel, the nanosized particles/monomers (a small
molecule) created find each other because of Brownian motion and then
stick together through the Van der Waals force. Then this process
continues, as more and more particles join together and form networks,
and networks of particles called aggregates join other aggregates,
thereby creating an aerosol gel, which consists of networks of fractal
aggregates. "Fractal aggregates are a scale invariant, random, loosely
packed ensemble of monomers which have assembled themselves from
initially separate entities."
R. Dhaubhadel Abstract |
 | Combustion - It is an exothermic reaction when large
amounts of heat and energy are released by the rapid oxidation of a
fuel. As opposed to burning, this is not as rapid. |
 | Volume Fraction - A very important issue in how
quickly the aerosol gel will gel is the volume fraction (Fv).
Generally, the bigger the Fv, the quicker the gelling time.
Where, Fv= Volume of the particles/ Volume of the System.
The following graph of comparing Fv and gel time shows two
things, one, that gel time decreases as Fv increases and
two, that the smaller the monomer radius, the faster the gel time.
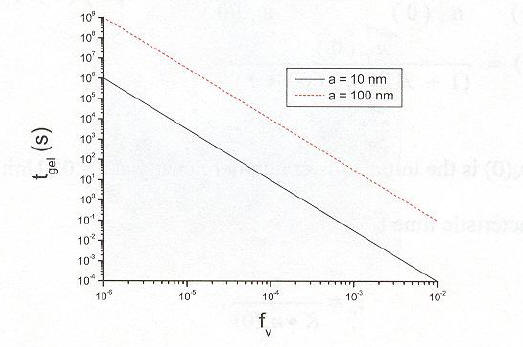
Our goal volume fraction is ~10-4. The reason being, if the
Fv is to small, the gel time is longer than the
gravitational settling time scale, and all of the nanosized monomers
will settle to the bottom of the container rather than aggregate.
Furthermore, if the Fv is too short, other time scales like
the rate of heat dissipation play an important role. Meaning, if the
monomers are still in a molten form and they start to aggregate, the
monomers will coalesce with other monomers and will not form a
beautiful network of fractal aggregates, but rather a goop made up of
the former monomers. |
 | Particle Size - The size of the constituents is
also very important on whether an aerosol will gel. As seen in the
above graph, having large particles results in a slower gelling time
after combustion. Having a fast gelling time is very practical. From
experiment, it has been shown that our combustion process creates
nanosized particles in the 10nm range. |
|
|



